TRANSLATION INHIBITION BY 4E-BP DURING INFECTION IS REQUIRED FOR BOOSTING ANTI-MICROBIAL PEPTIDE SYNTHESIS
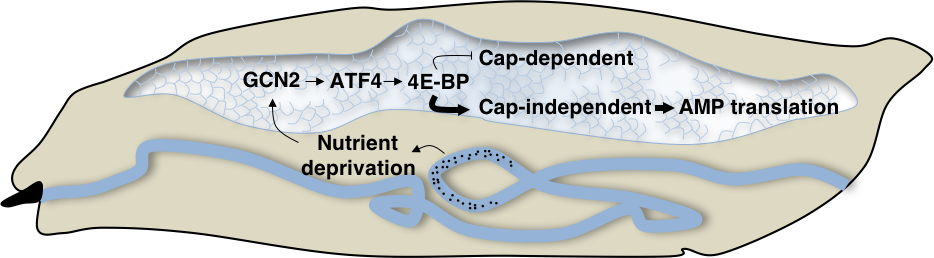
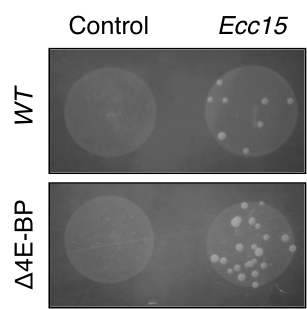
We found a surprising new role for the cellular stress response in regulating the innate immunity (PMC5728446). We discovered that the stress response transcription factor, ATF4, is induced by the amino acid sensing kinase, GCN2, during pathogenic infection in Drosophila. Lack of ATF4 or GCN2 results in an immune-compromised phenotype. We found that this phenotype is due to translational inhibition imposed by an ATF4 target, 4E-BP. Curiously, 4E-BP mutants were discovered to be immune-compromised in 1996 but the molecular mechanisms of the phenotype were unknown.
We figured out that 4E-BP is required for efficient translation of anti-pathogen agents (anti-microbial peptides or AMPs) required for eliminating pathogen. AMPs are able to bypass translational inhibition imposed by 4E-BP via their 5’ untranslated regions (UTRs). Not only are AMPs able to bypass translation inhibition by 4E-BP, they actively require 4E-BP function to bias cellular translation in their favor! This mechanism of imposing translation bias finally resolves the 20 year old mystery of why 4E-BP mutants are immune-compromised.
We are currently interested in figuring out on what features of the AMP 5’UTRs render them 4E-BP dependent and how GCN2 is able to sense bacterial infection.
